Background:
Soil salinization can greatly reduce crop yields leading to negative effects on human nutrition and livelihoods. Globally, predominantly in arid and semi-arid climates, it is estimated that 20% of irrigated land is negatively impacted by salt.1 Salts occur naturally in soils and water, however many factors can increase the concentration of salts in the soil to levels where plant growth is adversely affected. Furthermore, salinity decreases the overall health of a soil by destroying soil structure, reducing infiltration and conductance of water thereby increasing erosion potential, influencing soil pH which can in turn affect nutrient availability, and can contaminate drinking water.
Climate, soil parent material and subsequent rock weathering, groundwater, flooding of land by seawater, and equilibrium reactions control natural sources of salinity. For example, areas with a high water table and high rates of evapotranspiration can cause salt accumulation at the soil surface as water is lost to the atmosphere and salts are left behind.2 However, human influences can exacerbate salinization of soils. For example, the addition of soil amendments, including certain fertilizers can add salts to soils. Irrigation water can also be a source of salinity and over time, particularly in arid environments, as land is cyclically irrigated and water is lost through evapotranspiration, salts can accumulate in the soil, especially if leaching of salts is limited by climate or soil characteristics such as a low permeability layer.2 A salt stressed plant can also lead to plant nutrient deficiencies.3 Furthermore, depending on the type of ions present, for example a sodic soil defined by having high levels of sodium, can cause an increase in pH, which can also lead to limited nutrient availability within the soil solution.4

Sodic soil with degraded structure (marked as “Labeled for Reuse with Modification” on google search): https://pxhere.com/en/photo/362155
Diagnosis:
Physical characteristics can be the first step in diagnosing a salt affected soil. Low permeability indicated by ponding on the soil surface (sodic soils), loss of structure (sodic soils), and the development of salt crusts (saline and sodic soils) are all indicators of salt affected soils. However, where possible, field kits and lab tests can provide useful and more detail information that can help in determining the management strategy for remediation of the salt affected soil. Salt affected soils can be divided into three categories: saline, saline-sodic, and sodic. Depending on the category management may vary.
Saline
Saline soils describe soils with high enough soluble salts in the soil solution to negatively affect plant growth. These salts can be made up of calcium, magnesium, potassium, sodium, chloride, carbonate, and sulfate. Usually saline soils still have good physical structure and water infiltration and soil permeability is not affected. Specific physicochemical characteristics include:
- Electrical Conductivity: >4 ds m-1
- Exchangeable Sodium Percentage: <15%
- Sodium Adsorption Ratio: <13%
- pH: <8.5
- Visual: white crusts on soil
Sodic
Sodic soils are similar to saline soils, however the soil solution of sodic soils are dominated by sodium which tends to disperse soil particles leading to negative impacts on soil physical structure and reduced water infiltration or if clay particles are dispersed and leach to a lower layer, reduced permeability may be seen rather than reduced infiltration at the surface. Specific physicochemical characteristics include:
- Electrical Conductivity: <4 ds m-1
- Exchangeable Sodium Percentage: >15%³
- Sodium Adsorption Ratio: >13%
- pH: >8.5
- Visual: Dispersed, crusts, structure compromised, can be very dark in color
Saline-Sodic
Soils can be a combination of saline and sodic. Saline-sodic soils tend not to be afflicted with poor physical structure as their soil solution is concentrated with soluble salts that help floculate (not disperse) the soil. Specific physicochemical characteristics include:
- Electrical Conductivity: > 4 4 ds m-1
- Exchangeable Sodium Percentage: >15%
- Sodium Adsorption Ratio: >15%
- pH: <8.5
- Visual: Not dispersed, okay structure, not usually dark in color
Management:
Below is a short summary of various management practices. For a full list of additional resources click here.
Depending on the type of salts present in the soil, management will vary:
Saline soil:
Removing excess salts from the rooting zone is essential for healthy plant growth. Leaching salts with water is common, however, in areas with high water tables, drainage prior to leaching may be required. These practices mitigate the effect of salts in the rooting zone:
- Irrigation management to reduce salts in the soil, especially during seedling establishment. The rate of irrigation water application should be lower than the infiltration rate.
- Leaching salts below the root zone using the leaching fraction requirement equation
- Artificial drainage, leaching, and disposal of saline irrigation water. If draining is not possible, “vertical” farming can be implemented.
- Crop bed management to mitigate effects of salinity
- Planting more salt tolerant crops, such as barley
- Soil amendments to reduce evaporation help keep moisture in the soil and avoid concentrating salts at the surface
Example of differing furrow irrigation strategies on salt accumulation:
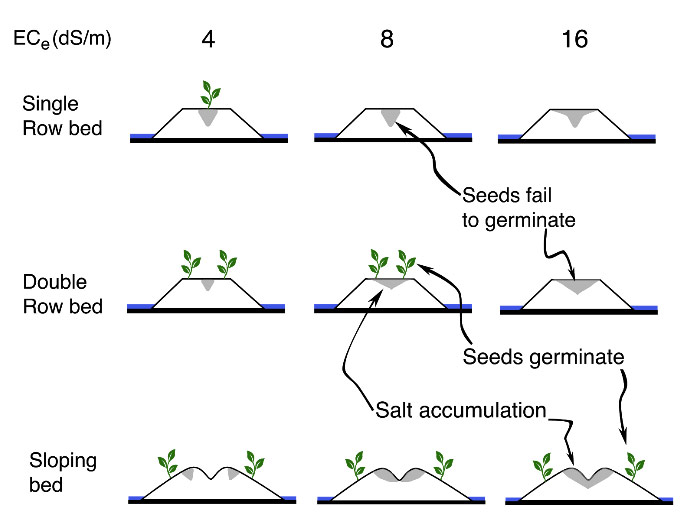
Figure 1: Crop bed configurations to mitigate negative effects of salinity on crop growth for varying levels of electrical conductivity (dS/m) (adapted from Bernstein et al. 1955).
Sodic soils:
Sodic soils differ from saline soils in that they have sodium on the exchange sites of the soil causing soils to disperse, lose structure, and prevent water from infiltrating or reduce the permeability of the soil. Sodium must first be removed from the exchange sites to allow for water to infiltrate and then the same management practices from saline soils can be applied to sodic soils:
- Addition of gypsum (CaSO4) to remove sodium (Na+) from the cation exchange sites on soil and replace with calcium (Ca2+) to aid in flocculating soil to improve water infiltration and movement.
- If soils are already high in calcium carbonate (lime), elemental sulfur or sulfuric acid can be added to the soil without the addition of calcium to lower the pH and dissolve the naturally occuring calcium carbonate in the soil, releasing the calcium and allowing it to exchange with the sodium on the soil cation exchange sites.
Saline-Sodic Soils:
Saline-sodic soils require that the excessive salts in solution and the sodium on the exchange sites both be addressed.
- Addition of gypsum (CaSO4) to remove sodium (Na+) from the cation exchange sites on soil and replace with calcium (Ca2+) to aid in flocculating soil to improve water infiltration and movement.
- If soils are already high in calcium carbonate (lime), elemental sulfur or sulfuric acid can be added to the soil without the addition of calcium to lower the pH and dissolve the naturally occuring calcium carbonate in the soil, releasing the calcium and allowing it to exchange with the sodium on the soil cation exchange sites.
- Once sodium is successfully removed from the exchange sites and the infiltration and permeability of the soil is improved, irrigating with good quality (low sodium and salts) irrigation water can leach excess salts from the soil.
- Other management practices from saline soils can be applied to saline-sodic soils once soil structure is improved.
Additional Resources:
Leaching Salts
- An Extension Bulletin by USDA on leaching and irrigation management to mitigate the negative impacts on salt https://soilhealth.ucdavis.edu/download_file/view/229/224
- An Extension Bulletin by Colorado State University summarizing different methods to reduce the negative impacts of salt on crop growth, including irrigation management and salt tolerant crops https://soilhealth.ucdavis.edu/download_file/view/237/224
- FAO handbook on using saline water for irrigation http://www.fao.org/docrep/T0667E/T0667E00.htm
- UC ANR publication on drip irrigation management for saline soils https://soilhealth.ucdavis.edu/download_file/view/238/224
- UC ANR publication on managing salts by leaching including details on the leaching requirement https://soilhealth.ucdavis.edu/download_file/view/229/224
Saline vs. Sodic Soils:
- Extension bulletin by North Dakota State University on saline vs. sodic soils and their respective management https://soilhealth.ucdavis.edu/download_file/view/239/224
- Website by the Government of Western Australia on managing sodic soils https://www.agric.wa.gov.au/dispersive-and-sodic-soils/managing-waterlogged-dispersive-sodic-soils
- Extension bulletin by UC ANR on how to manage salt affected soils https://anrcatalog.ucanr.edu/pdf/8519.pdf
Salt Tolerant Crops
- A chapter on “Crops in Saline Soils” from the handbook for managing salinity produced by the FAO http://www.fao.org/docrep/x5871e/x5871e04.htm#3.3%20Crops%20in%20saline%20soils
- CGIAR bulletin on salt tolerant crops https://soilhealth.ucdavis.edu/download_file/view/240/224
- FAO Annex on crop salt tolerance with a table of salinity tolerance for varying crops http://www.fao.org/docrep/005/y4263e/y4263e0e.htm
Further Information
- A handbook from International Irrigation Management Institute on salinity in developing countries at different scales (field, regional, national) https://soilhealth.ucdavis.edu/download_file/view/241/224
- The FAO website for soils with particular reference to salt affected soils http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/en/
- FAO handbook on managing salinity in soils http://www.fao.org/docrep/x5871e/x5871e00.htm#Contents
- FAO handbook on irrigation water management with a section for saline soils http://www.fao.org/docrep/r4082e/r4082e00.htm#Contents
- FAO handbook on saline soils in the Eurasian Region https://soilhealth.ucdavis.edu/download_file/view/242/224
- FAO handbook on irrigating with low quality water in agriculture http://www.fao.org/docrep/003/T0234E/T0234E00.HTM
- CGIAR report on the reuse of saline irrigation water https://wle.cgiar.org/content/making-case-reusing-saline-water-and-restoring-salt-affected-agricultural-lands
- CGIAR handbook for managing salinity in Iran https://soilhealth.ucdavis.edu/download_file/view/243/224
- CGIAR open access article on agroforestry in India and it’s rehabilitative effects on saline soils https://cgspace.cgiar.org/handle/10568/45637
- Website on soil salinity by the Government of Western Australia, predominantly highlights field and regional scale descriptions of the problems and some solutions https://www.agric.wa.gov.au/climate-land-water/soils/managing-soils/soil-salinity
- Worldwatch Institute blog on vertical farms in Kenya http://blogs.worldwatch.org/nourishingtheplanet/vertical-farms-finding-creative-ways-to-grow-food-in-kibera-africa-agriculture-farmers-food-security-hunger-income-kenya-urban-farming-kiberia-urban-harvest-women-vertical-farms-soladarites-red-cross/
- Extension bulletin by Colorado State University on crop bed and irrigation management for mitigating negative effects of salinity https://soilhealth.ucdavis.edu/download_file/view/237/224
References:
- Sairam, R.K., Tyagi, A., 2005. Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 10, 615–620. https://doi.org/10.1016/j.tplants.2005.10.002
- Daliakopoulos, I.N., Tsanis, I.K., Koutroulis, A., Kourgialas, N.N., Varouchakis, A.E., Karatzas, G.P., Ritsema, C.J., 2016. The threat of soil salinity: A European scale review. Sci. Total Environ. 573, 727–739. https://doi.org/10.1016/j.scitotenv.2016.08.177
- Grattan, S.R., Grieve, C.M., 1999. Salinity - mineral nutrient relations in horticultural crops. Sci. Hortic. (Amsterdam). 78, 127–157.
- Food and Agriculture Organization of the United Nations. 1988. Salt-Affected Soils and their Management. Yadav, J.S.P., Massoud, F.I. Chapter 4: Soidc Soils and their Management. http://www.fao.org/docrep/x5871e/x5871e00.htm#Contents
- Horneck, D. A., J. W. Ellsworth, B. G. Hopkins, D. M. Sullivan, and R. G. Stevens. "Managing Salt Affected Soils for Crop Production." A Pacific Northwest Extension Publication (2007): n. pag. Oregon State University Extension Program. Web. <http://extension.oregonstate.edu/catalog/pdf/pnw/pnw601-e.pdf>.
- Brady, Nyle C., Ray R. Weil, and Nyle C. Brady. "Soil Acidity, Alkalinity, and Salinity." Elements of the Nature and Properties of Soils. 2nd ed. Upper Saddle River, NJ: Prentice Hall, 2004. N. pag. Print.
- Food and Agriculture Organization of the United Nations. 1992. The use of saline waters for crop production – FAO irrigation and drainage paper 48. Rhoades, J.D., Kandiah, A., Mashali, A.M. Chapter 6: Management principles and practices for safe use of saline water. http://www.fao.org/docrep/t0667e/t0667e0b.htm
- Bernstein, L., Fireman, M. and Reeve, R.C. 1955. Control of salinity in the Imperial Valley, California. USDA, ARS 41-4, 14 p.
